| CAS
NO. |
4635-59-0 |
|
| EINECS
NO. |
225-059-1 |
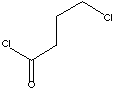
| FORMULA |
Cl(CH2)3COCl |
| MOL
WT. |
140.99 |
|
H.S.
CODE
|
|
|
TOXICITY
|
|
| SYNONYMS |
4-Chlorobutyryl chloride; Chlorobutanoic acid chloride;
|
| a-Chlorobutyroyl chloride; 4-Chlorobutyroyl
chloride; gamma-Chlorobutyryl chloride; 4-Chlorobutyroyl Chloride; |
| SMILES |
|
|
CLASSIFICATION
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
clear
liquid |
| MELTING POINT |
-47
C |
| BOILING
POINT |
173 - 174 C |
| SPECIFIC GRAVITY |
1.258 |
| SOLUBILITY
IN WATER |
hydrolyzes |
| pH |
|
| VAPOR DENSITY |
4.86 |
| AUTOIGNITION |
440
C
|
| NFPA RATINGS |
|
| REFRACTIVE
INDEX
|
1.4610-1.4620 |
| FLASH
POINT |
85
C |
| STABILITY |
Stable
under ordinary conditions. |
|
APPLICATIONS
|
|
Acid chlorides are used as very reactive intermediates to prepare carboxylic
acid derivatives including anhydrides, esters and amides because of the two
strong electron withdrawing chlorine and oxygen on the carbonyl compound, and
positive charge carbon accordingly. It is easy for a weak nucleophile to attack
the carbon. Acid chlorides are also reactive with Gilman reagents to prepare
large molecules from small ones by replacing the halides with an organic group.
4-Chlorbutyryl chloride, an acid chlorides with
an additional chloride at the terminal carbon, is
a clear to yellowish liquid with a pungent odor; melting
point -47 C. It hydrolyses with water. It
must be protected against moisture, air, light and
heat during storage and handling. It is used in
the preparation of pharmaceuticals, agrochemicals, peroxides
and other target molecules. |
| SALES
SPECIFICATION |
|
APPEARANCE
|
clear
liquid |
|
ASSAY |
98.0%
min
|
|
MOISTURE |
0.05%
max
|
|
COLOR,
AHPA |
50
max |
| TRANSPORTATION |
| PACKING |
250kgs
in drum |
| HAZARD CLASS |
6.1
((Packing Group: I) |
| UN
NO. |
2927 |
| OTHER
INFORMATION |
|
Hazard
Symbols: C, Risk Phrases: 22,34 |
|
GENERAL
DESCRIPTION OF ACYL HALIDE
|
|
Acyl is a radical formed from an organic acid by removal of a hydroxyl group.
The general formula of acyl compound is RCO-. Acyl halide is one of a large group of organic
substances containing the halocarbonyl group, have the general formula RCO·X,
where X is a halogen atom
(fluorine, chlorine, bromine, iodine, and astatine) and R may be aliphatic, alicyclic, aromatic,
and H etc. In substitutive chemical nomenclature, their names are
formed by adding '-oyl' as a suffix to the name of the parent compound; ethanoyl
chloride, CH3COCl, is an example. The terms acyl and aroyl halides refer to aliphatic or aromatic derivatives,
respectively. Acyl halides are made by replacing the -OH
group in carboxylic acids by halogen using halogenating agents. They react
readily with water, alcohols, and amines and are widely used in organic
synthetic process whereby the acyl group is incorporated into the target
molecules by substitution of addition-elimination sequence called acylation
reaction. Acylation reaction involves substitution by an electron donor
(nucleophile) at the electrophilic carbonyl group (C=O). Common nucleophiles in the acylation reaction are aliphatic and
aromatic alcohols, both of which give rise to esters and
amines (RNH2)
which give amides. The carboxylic acid (X = OH) itself can
function as an acylating agent when it is protonated by a strong acid catalyst
as in the direct esterification of an alcohol. Two common acylation agents, with
the general formula RCOX, are acid halides (X = halogen atom) and anhydrides (X
= OCOR). Schotten-Baumann reaction is an acylation reaction that uses an acid chloride in
the presence of dilute alkali to acylate the hydroxyl and amino group of organic
compounds. There are also other acylating agents. Acyl halide compounds are
used as an intermediate for agrochemicals and pharmaceuticals,
dyes, and peroxide compounds as well as textile
auxiliaries, emulsifiers. |
